AniRA Vectorologie

PRÉSENTATION
AniRA-Vectorologie est intégrée dans la plateforme régionale AniRA labélisée IBiSA, membre de l’Infrastructure de Recherche nationale Celphedia.


Le plateau technique AniRA-Vectorologie propose à la communauté scientifique (académique lyonnaise, régionale ou nationale et privée) un savoir-faire technologique dans le domaine de la production de vecteurs retroviraux et lentiviraux destinés au transfert stable et efficace de séquences nucléiques (gène, ADNc, Sh/miARN) dans des lignées cellulaires, des cellules primaires difficilement transfectables (cellules souches, …) ou dans un organisme entier. Ce système de transfert est utilisé dans de nombreux domaines qui vont de la recherche fondamentale à la clinique. Les applications sont multiples :
- La surexpression de gènes ou l’expression ectopique de gènes
- Le knock-down de gènes par des vecteurs véhiculant des shRNAs
- L’expression ou le knock down régulé de gènes par des vecteurs lentiviraux inductibles
- L’édition du génome (CRISPR/Cas9)
La plateforme de vectorologie offre une expertise dans la production de lots de vecteurs rétro et lentiviraux bien caractérisés et prêts à l’emploi en confinement C2. Ces productions sont réalisées dans le respect strict des conditions de biosécurité édictées par le Comité d’expertise des utilisations confinées d’OGM CEUCO et le ministère de l’enseignement supérieur de la recherche et de l’innovation MESRI (confinement C2 ou C3 pour la production) selon une chartre d'utilisation des services propre à la plateforme de vectorologie. La principale activité de la plateforme de vectorologie est la production de vecteurs sur la base de MLV, HIV-1 ou SIV.
Citation de la SFR Biosciences sur les publications
ACTIVITÉS
- Conseil aux utilisateurs dans le choix des vecteurs et pour le niveau de biosécurité des vecteurs.
- Production de lots de vecteurs rétro et lentiviraux en confinement L2 ou L3
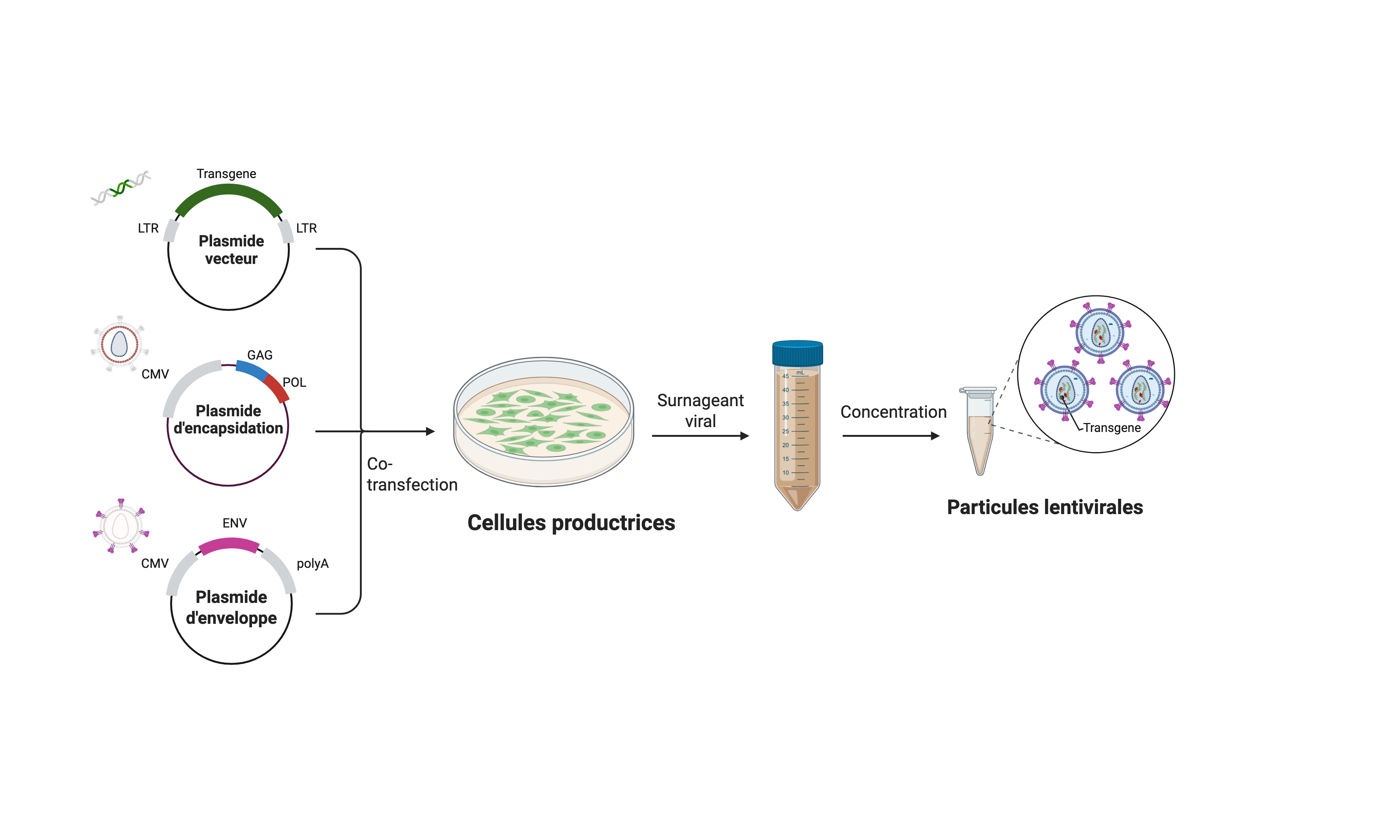
La production des vecteurs lentiviraux est réalisée par tri-transfection en suivant la méthode de transfection au phosphate de calcium, dans une lignée de cellules embryonnaires de rein (293T). Les trois plasmides utilisés codent respectivement pour :
- La glycoprotéine d’enveloppe G du virus de la stomatite vésiculaire (VSV-G).
- Les protéines de structures et enzymatiques du virus (gag+pol).
- Le vecteur viral codant pour un gène d’intérêt (GFP, …).
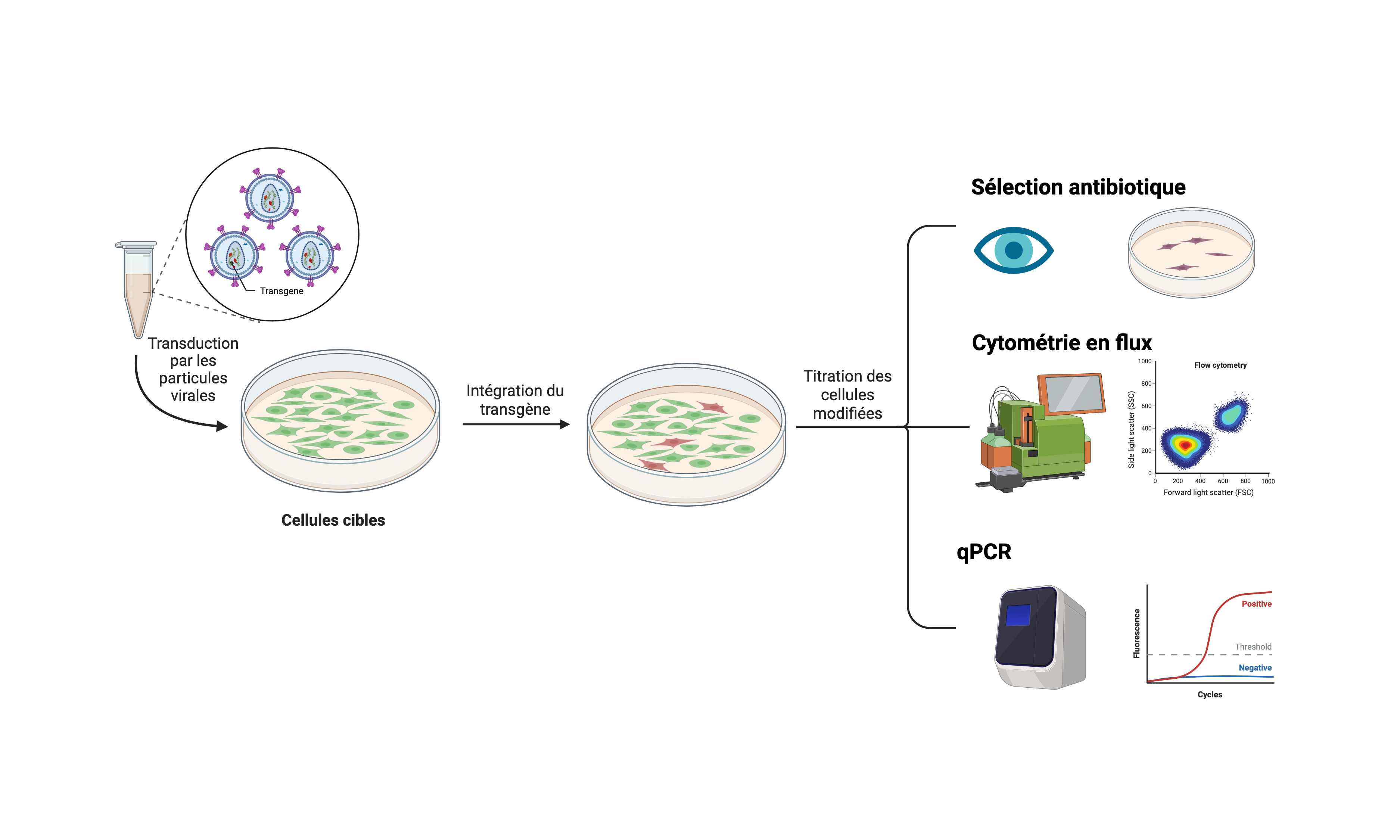
- Titration de particules virales infectieuses
Par transduction de cellules HEK293T suivie de :
- Détection par cytométrie en flux (FACS), du vecteur grâce à un gène rapporteur codant pour un fluorochrome (GFP, mRFP, mKate,…),
- Sélection avec un antibiotique (puro, néo, zéo,…) grâce à un gène de résistance présent dans le vecteur.
- Quantification du nombre de copies de génome intégré par QPCR (si les séquences rétrovirales sont compatibles avec nos conditions).
Règlement Intérieur du plateau
CONTACT/LOCALISATION
La plateforme est hébergée par le CIRI (Centre International de Recherche en Infectiologie) U1111/UMR5308 Inserm-CNRS-UCBL - ENS de Lyon dans l'équipe EVIR (Virus enveloppés, vecteurs et immunothérapie) située au rez-de-chaussée du M5 (ex LR5) de l'ENS de Lyon Site Monod :
Bat. M5 – Équipe : EVIR
École Normale Supérieure de Lyon
9 rue du Vercors
69007 Lyon
Pour tout renseignement, contactez :
Caroline Costa Fejoz : responsable plateau
Tel : 04 72 72 87 31
Mail : plateau.vectorologie@ens-lyon.fr


