AniRA Vectorology
PRESENTATION
AniRA-Vectorologie is integrated into the regional AniRA platform labeled IBiSA , member of the national Research Infrastructure Celphedia.


The AniRA-Vectorologie technical platform offers technological know-how in the field of production of retroviral and lentiviral vectors for stable and efficient transfer of nucleic acid sequences (gene, cDNA, Sh/miRNA) into cell lines, difficult to transfect primary cells (stem cells, etc.), or into entire organisms. This transfer system is used in many fields ranging from fundamental research to clinical practice. The applications include:
- Gene overexpression or ectopic gene expression
- Gene knockdown by vectors carrying shRNAs
- Regulated gene expression or knockdown by inducible lentiviral vectors
- Genome editing (CRISPR/Cas9)
The vectorology platform offers expertise in the production of batches of well-characterised and ready-to-use retroviral and lentiviral vectors in C2 containment. These productions are carried out in strict compliance with the biosafety conditions set out by the Expert Committee for the Contained Use of GMOs CEUCO and the Ministry of Higher Education, Research and Innovation MESRI (C2 or C3 containment for production) according to a charter for the use of services specific to the vectorology platform. Our main activity is the production of vectors based on MLV, HIV-1 or SIV.
ACTIVITIES
- Advise users for the choice of vectors and the level of biosecurity required.
- Production of batches of retro and lentiviral vectors in L2 or L3 containment
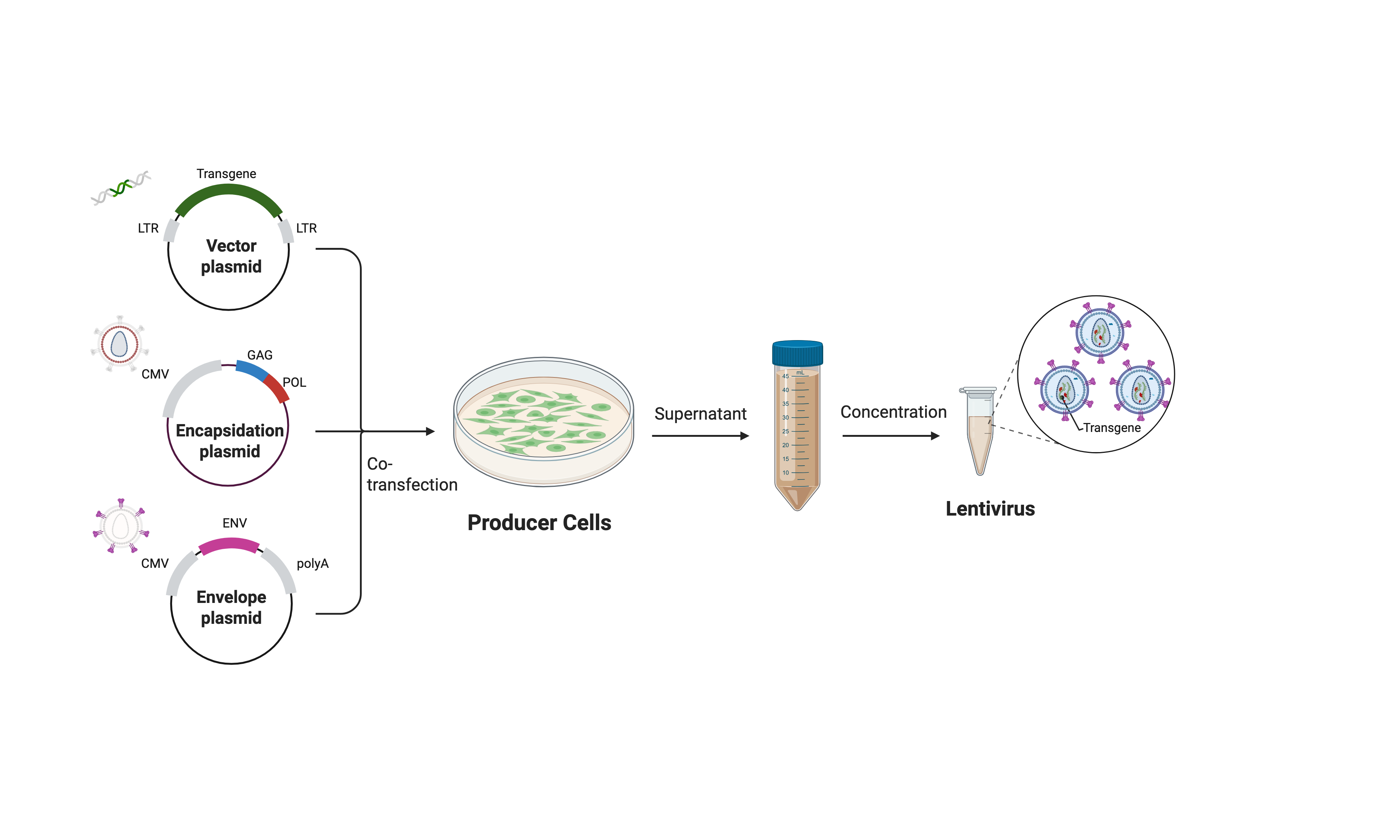
The production of lentiviral vectors is carried out by tri-transfection using calcium phosphate transfection method, in an embryonic kidney cell line (293T). The three plasmids used code respectively for:
- Vesicular stomatitis virus envelope glycoprotein G (VSV-G).
- The structural and enzymatic proteins of the virus (gag+pol).
- The viral vector coding for a gene of interest (GFP, etc.)
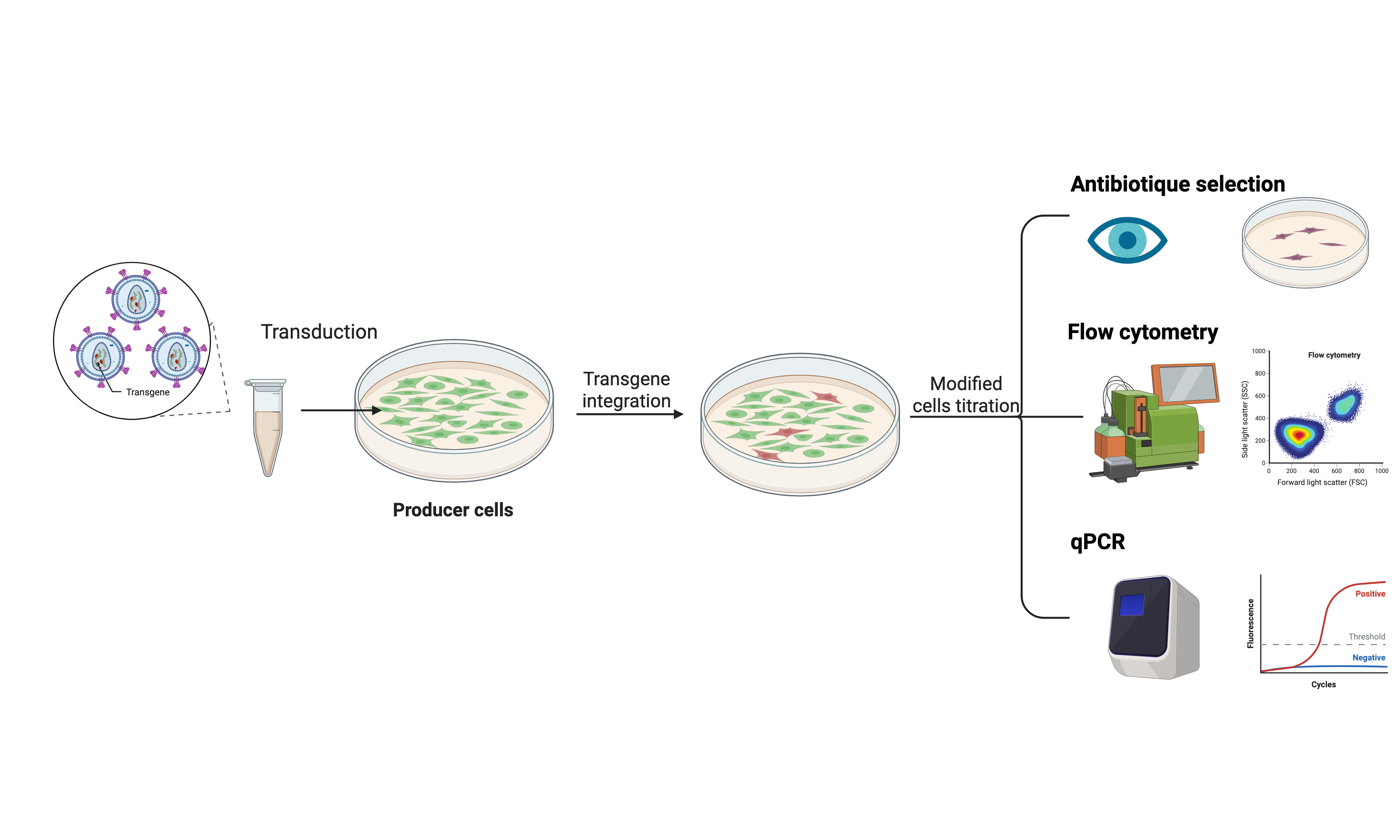
- Titration of infectious viral particles
By transduction of HEK293T cells followed by:
- Detection by flow cytometry (FACS) of the vector using a reporter gene coding for a fluorochrome (GFP, mRFP, mKate, etc.),
- Selection with an antibiotic (puro, neo, zeo, etc.) thanks to a resistance gene present in the vector.
- Quantification of the number of copies of the integrated genome by QPCR (if the retroviral sequences are compatible with our conditions).
Operating procedures of the facility
CONTACT/LOCATION
The platform is hosted by the CIRI (International Center for Infectious Disease Research) U1111/UMR5308 Inserm-CNRS-UCBL - ENS de Lyon in the EVIR team (Enveloped viruses, vectors and immunotherapy) located on the ground floor of M5 (ex LR5) of the ENS de Lyon Monod Site:
Bat. M5 – Team: EVIR
Lyon Higher Normal School
9 rue du Vercors
69007 Lyon
For information, contact:
Caroline Costa Fejoz: facility manager
Tel: 04 72 72 87 31
Email: plateau.vectorologie@ens-lyon.fr


